- Bamlanivimab (LY-CoV555) has greater affinity and potency relative to other RBD-binding and ACE2-blocking
antibodies tested in this study
- Because of its potency, bamlanivimab provides a therapeutic foundation to be administered with another antibody
to expand the protection against viral variants
- Study was the first of its kind to show a neutralizing antibody can decrease SARS-CoV-2 viral shedding and
transmission by blocking virus replication in the upper airway
- Bamlanivimab moved from first screen to clinical testing in 90 days1 and is the world’s first
monoclonal antibody specifically developed against SARS-CoV-2 to receive FDA Emergency Use Authorization
(EUA)2
- Since EUA, bamlanivimab has been used to treat approximately 400,000 high-risk COVID-19 patients in the U.S.
alone and has been authorized in more than 15 countries
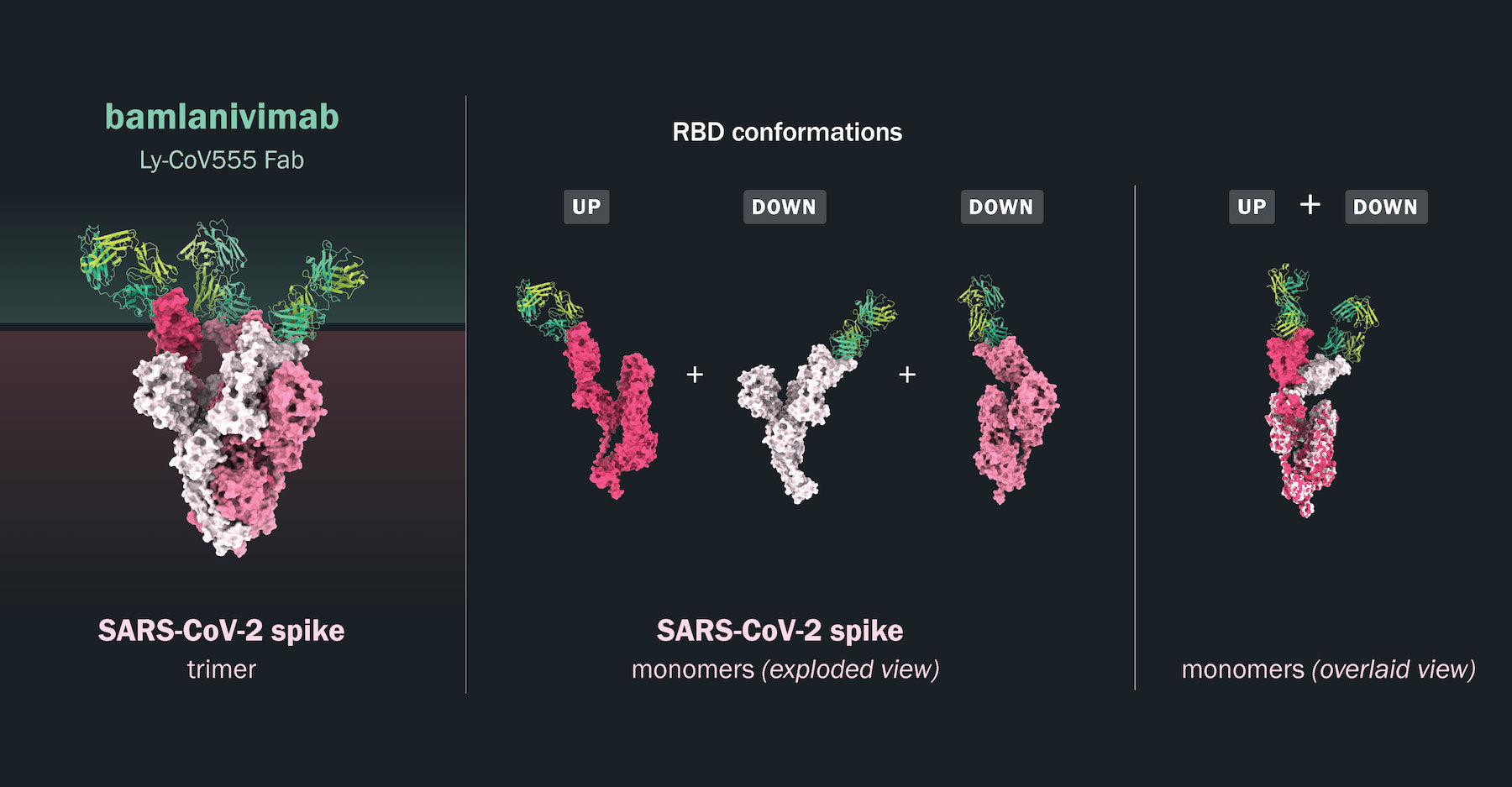
The unique binding of bamlanivimab to the SARS-CoV-2 spike protein: The spike protein
exists as a trimer of three identical monomers on the surface of the SARS-CoV-2 virus. Structural modeling (left
panel) of the spike trimer in shades of pink and white is shown with the target-binding fragments (Fabs) of
bamlanivimab (in green and yellow) bound to the RBD of the spike protein. This analysis shows three bamlanivimab
Fab fragments bound to one spike trimer. One of the spike proteins is in the up position (dark pink) with the
other two in the down position (light pink and white). The middle panel shows an isolated view of the spike
monomers (dark pink, white and light pink) with the bound bamlanivimab Fab fragments in green and yellow. In the
right panel, two spike monomers bound in the up and down positions by the bamlanivimab Fab fragments are overlaid.
3D structural model provided by JS McLellan Group, University of Texas.
VANCOUVER, British Columbia--(BUSINESS WIRE)--
AbCellera (Nasdaq: ABCL) and collaborators today announced the publication of research in Science Translational Medicine characterizing the high potency of bamlanivimab
(LY-CoV555) to neutralize SARS-CoV-2 by uniquely binding both the up and down confirmations of the spike
receptor-binding domain (RBD) and inhibiting critical interactions with the angiotensin converting enzyme 2 (ACE2)
cellular receptor necessary for viral entry. Data generated in a preclinical model showed prophylactic treatment
with bamlanivimab resulted in significant decreases in viral load and replication in the upper and lower respiratory
tracts after SARS-CoV-2 exposure, indicating the potential of bamlanivimab to reduce viral shedding and
transmission. These data, which were generated prior to initiating clinical trials in June 2020 and published today,
support the observed substantial clinical efficacy of bamlanivimab in treating and preventing COVID-19.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210405005386/en/
Previously Reported Clinical Trial Results
Bamlanivimab has been evaluated both alone and together with other antibodies in more than 5,000 patients across
multiple clinical trials and is currently authorized in more than 15 countries. Bamlanivimab alone versus placebo
has been shown to reduce hospitalization by 70% in high-risk patients with early COVID-19 infection3 and
reduce the risk of contracting COVID-19 by up to 80% in nursing home residents when used as a
prophylactic.4
Because of its potency, bamlanivimab also provides a therapeutic foundation to be administered with other antibodies
to expand the protection against viral variants. The first of these, bamlanivimab together with etesevimab, has been
authorized in the U.S. and within the European Union, and Phase 3 data show that this antibody therapy reduces
COVID-19-related hospitalizations and death by 87%.5 Most importantly, across all the clinical trials,
all COVID-19-related deaths occurred in patients taking the placebo; no deaths occurred in patients who received an
antibody therapy, either bamlanivimab alone or together with another antibody.5
“At the beginning of our pandemic response to COVID-19, we made a decision with our partners and collaborators to
develop a single antibody, emphasizing speed and scalability so that we would be able to help as many people as
possible,” said Carl Hansen, Ph.D., CEO and President of AbCellera. “Over the past four months, bamlanivimab has
been used to treat hundreds of thousands of people across the world -- more than any other COVID-19 antibody
therapy. We believe this has kept thousands of people out of the hospital, reducing the burden on our healthcare
systems, and, most importantly, has saved thousands of lives.”
Discussion of Data Published Today in Science Translational Medicine
Data from multiple in vitro assays of the 24 lead antibodies identified by AbCellera and collaborators
indicated bamlanivimab displayed greater neutralization potency despite similar RBD-binding affinities, suggesting
bamlanivimab has a unique binding profile to the SARS-CoV-2 spike protein. Structural analysis using X-ray
crystallography and electron microscopy demonstrated that bamlanivimab binds to an area on the spike protein
overlapping the ACE2 binding site that is predicted to be fully accessible in both the up and down conformations.
The RBD portion of the spike protein is the primary target for virus neutralization as it mediates the conserved
mechanism of viral entry to infect cells. The spike exists in an up or down position, with the up position enabling
interaction with the ACE2 receptor and the down position potentially contributing to immune system evasion.
Regardless of the state of the spike protein, bamlanivimab has high binding potency to the RBD of SARS-CoV-2 spike
protein.
“The unique ability of bamlanivimab to bind the spike protein in both the up and down position could underlie
bamlanivimab’s greater neutralization potency compared to other antibodies,” said Bo Barnhart, Ph.D., Scientific
Director at AbCellera. “These preclinical data show that modest doses of bamlanivimab provided protection against
SARS-CoV-2 infection, which has since been confirmed in clinical trials to protect residents and staff in long-term
care facilities and nursing homes. Neutralizing antibodies, like bamlanivimab, are designed to protect our most
vulnerable populations for whom vaccines are less effective. These data with bamlanivimab further confirm that
neutralizing antibodies have the potential to reduce SARS-CoV-2 viral transmission and prevent infection and can
provide immediate benefit when a life-saving treatment is needed.”
To determine the potential of neutralizing antibodies to prevent SARS-CoV-2 infection, nonhuman primates (NHPs) were
prophylactically treated with 1, 2.5, 15, or 50 mg/kg of bamlanivimab 24 hours prior to viral challenge. Critically,
viral replication as well as viral load were significantly reduced in the upper respiratory tract on Day 1 at
multiple doses. Additionally, viral load and replication were significantly reduced or undetectable in the lower
respiratory tract at several doses. At doses of 2.5 mg/kg and higher serum concentrations were associated with
maximal protection in this model.
“The data published today give insights into why bamlanivimab is so potent and further support all of our clinical
experience and data showing that bamlanivimab is a safe and effective therapy to treat and prevent COVID-19, when
administered early in the course of infection,” said Ester Falconer, Ph.D., Chief Technology Officer at AbCellera
and senior author of the paper. “Furthermore, bamlanivimab’s unique potency allows for lower dosing and enables
administration with another antibody to address SARS-CoV-2 variants. Over the past year, we have continued to screen
patient samples, identifying thousands of human antibodies and generating massive amounts of information about how
the human immune system responds to COVID-19. We have tracked the variants closely and identified a next-generation
antibody that is predicted to neutralize all circulating variant strains of concern of SARS-CoV-2. This antibody,
currently referred to as 1404, moved into preclinical development and manufacturing in January with our partner, Eli
Lilly and Company, and we are continuing to work closely with them and our collaborators for rapid advancement.”
The preclinical data for bamlanivimab was published online today in Science Translational Medicine and can be
found at: https://stm.sciencemag.org/content/early/2021/04/05/scitranslmed.abf1906.
About AbCellera’s Response to COVID-19
Bamlanivimab was developed from an antibody that was discovered from the blood of a recovered COVID-19 patient using
AbCellera’s pandemic response platform, in partnership with the Vaccine Research Center (VRC) at National Institute
of Allergy and Infectious Diseases (NIAID). Within one week of receiving the sample, AbCellera screened over five
million antibody-producing cells to identify and isolate approximately 500 unique antibodies that bind to
SARS-CoV-2, the virus that causes COVID-19. The binding antibodies were then tested by AbCellera, the VRC, and Eli
Lilly and Company (Lilly) to find those most effective in neutralizing the virus. Bamlanivimab was selected as the
lead candidate from this group of antibodies and was the first therapeutic candidate specifically developed against
SARS-CoV-2 to enter human clinical trials in North America. Bamlanivimab was the first monoclonal antibody to
receive EUA from the FDA and is currently being assessed in several clinical trials alone and together with other
antibodies.
AbCellera’s pandemic response capabilities were developed over the past three years as part of the Defense Advanced
Research Projects Agency (DARPA) Pandemic Prevention Platform (P3) program. The goal of the P3 program is to
establish a robust technology platform for pandemic response capable of developing field-ready medical
countermeasures within 60 days of isolation of an unknown viral pathogen. AbCellera’s ongoing efforts to respond to
the pandemic have identified more than 2,300 unique anti-SARS-CoV-2 human antibodies from multiple patient samples.
These antibodies are in various stages of testing by AbCellera and its partners.
About Bamlanivimab (LY-CoV555)
Bamlanivimab is a recombinant, neutralizing human IgG1 monoclonal antibody (mAb) directed against the spike protein
of SARS-CoV-2. It is designed to block viral attachment and entry into human cells, thus neutralizing the virus.
Bamlanivimab emerged from the collaboration between Lilly and AbCellera to create antibody therapies for the
prevention and treatment of COVID-19. Lilly scientists rapidly developed the antibody in less than three months
after it was discovered by AbCellera and the scientists at NIAID VRC. It was identified from a blood sample taken
from one of the first U.S. patients who recovered from COVID-19.
Lilly has successfully completed a Phase 1 study of bamlanivimab in hospitalized patients with COVID-19
(NCT04411628). A Phase 2/3 study in people recently diagnosed with COVID-19 in the ambulatory setting (BLAZE-1,
NCT04427501) is ongoing. A Phase 3 study of bamlanivimab for the prevention of COVID-19 in residents and staff at
long-term care facilities (BLAZE-2, NCT04497987) is also ongoing. In addition, bamlanivimab is being tested in the
National Institutes of Health-led ACTIV-2 study in ambulatory COVID-19 patients.
Bamlanivimab alone and together with etesevimab are authorized under special/emergency pathways, in the context of
the pandemic, in the U.S. and the European Union. In addition, bamlanivimab alone is authorized for emergency use in
Canada, Panama, Kuwait, the UAE, Israel, Rwanda, Morocco and numerous other countries. Through Lilly’s work with the
Bill & Melinda Gates Foundation, Lilly is providing doses of bamlanivimab free of charge in Rwanda and Morocco.
About AbCellera Biologics Inc.
AbCellera is a technology company that searches, decodes, and analyzes natural immune systems to find antibodies
that its partners can develop into drugs to prevent and treat disease. AbCellera partners with drug developers of
all sizes, from large pharmaceutical to small biotechnology companies, empowering them to move quickly, reduce cost,
and tackle the toughest problems in drug development. For more information, visit www.abcellera.com.
AbCellera Forward-looking Statements
This press release contains forward-looking statements, including statements made pursuant to the safe harbor
provisions of the Private Securities Litigation Reform Act of 1995. The forward-looking statements are based on
management’s beliefs and assumptions and on information currently available to management. All statements contained
in this release other than statements of historical fact are forward-looking statements, including statements
regarding our ability to develop, commercialize and achieve market acceptance of our current and planned products
and services, our research and development efforts, and other matters regarding our business strategies, use of
capital, results of operations and financial position, and plans and objectives for future operations.
In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,”
“expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,”
“ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking
statements contain these words. These statements involve risks, uncertainties and other factors that may cause
actual results, levels of activity, performance, or achievements to be materially different from the information
expressed or implied by these forward-looking statements. These risks, uncertainties and other factors are described
under "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and
elsewhere in the documents we file with the Securities and Exchange Commission from time to time. We caution you
that forward-looking statements are based on a combination of facts and factors currently known by us and our
projections of the future, about which we cannot be certain. As a result, the forward-looking statements may not
prove to be accurate. The forward-looking statements in this press release represent our views as of the date
hereof. We undertake no obligation to update any forward-looking statements for any reason, except as required by
law.
Source: AbCellera Biologics Inc.

View source version on businesswire.com:
https://www.businesswire.com/news/home/20210405005386/en/
Inquiries
Media: Jessica Yingling, Ph.D.; media@abcellera.com, +1(236)521-6774
Business Development: Kevin Heyries, Ph.D.; bd@abcellera.com, +1(604)559-9005
Investor Relations: Melanie Solomon; ir@abcellera.com, +1(778)729-9116
Source: AbCellera Biologics Inc.