Under DARPA’s Pandemic Prevention Platform (P3) program, AbCellera will apply its state-of-the art capabilities in human antibody discovery and immune profiling to establish rapid countermeasures for viral pandemics.
Vancouver, Canada (March 13, 2018) - AbCellera Biologics Inc. announced today that it was awarded a contract from the Defense Advanced Research Projects Agency (DARPA) to develop rapid countermeasures against viral outbreaks. Over the four-year contract, AbCellera will receive up to USD $30 million in funding to establish an end-to-end platform for rapid pandemic response, and will lead an internationally recognized team of experts in virology, antibody discovery, and gene therapy.
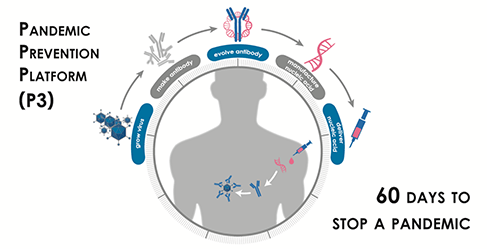
The project is part of the Pandemic Prevention Platform (P3), a high-priority initiative of DARPA’s Biological Technology Office. The P3 program seeks to develop a robust technology platform for pandemic response capable of developing field-ready medical countermeasures within 60 days of isolation of a viral pathogen. To achieve this ambitious goal, AbCellera and its partners will develop and integrate innovative technologies for viral culture and production, rapid human antibody discovery, protein engineering, and delivery of nucleic acid-encoded antibodies as prophylactic protection against viral infection. AbCellera’s platform development and testing will include the discovery of thousands of human antibodies against a wide array of influenza strains and validation using a variety of other high-priority viral pathogens. In addition to the proposal submitted by the AbCellera-led team, the P3 program has funded three other consortia led by Medimmune, the Duke Human Vaccine Institute, and Vanderbilt University Medical Center.
Carl Hansen, founding CEO of AbCellera, commented: “Through the P3 program, DARPA has set a bold vision to establish effective response capabilities for viral threats. The recent Ebola and Zika pandemics have made it clear that we are not equipped to deal with viral pandemics. The severity of seasonal flu this year is a sobering reminder that viral outbreaks present a serious risk to public health for which we must be better prepared. We are honoured to lead a team to help achieve the important goals of the P3 program.”
Col. Matthew Hepburn, the DARPA P3 Program Manager, noted in DARPA’s original announcement of the program: “We need to be able to move at this speed considering how quickly viral outbreaks can get out of control. The technology needs to work on any viral disease, whether it’s one humans have faced before or not. If we’re successful, DARPA could take viral infectious disease outbreaks off the table.”
DARPA, founded in 1958, is an agency of the U.S. Department of Defense. Through collaborations with academic, industry, and government partners, DARPA makes investments across multiple sectors to drive breakthrough technologies for U.S. national security.
An announcement of the P3 program was made earlier by DARPA: http://www.darpa.mil/news-events/2018-02-22
About AbCellera Biologics Inc.
AbCellera is a privately held company that engages in partnerships to discover and develop next-generation therapeutic antibodies. AbCellera’s single-cell platform integrates end-to-end capabilities for therapeutic antibody discovery through a combination of technologies including proprietary immunizations, microfluidics, high-throughput imaging, genomics, computation, and laboratory automation. Ultra-deep screening of single B cells allows unprecedented access to natural immune responses, enabling rapid isolation of large and diverse panels of high-quality lead antibodies from any species, including humans.
Contact
Kevin Heyries, PhD Telephone: 604.559.9005 Email: media@abcellera.com Web: www.abcellera.com